Prescribing Information
touchINSIGHT Gocovri® for the treatment of dyskinesia and OFF episodes
Understand how amantadine extended release (Gocovri) represents a significant advance in the treatment of dyskinesia and OFF in Parkinson’s disease
Report
touchINSIGHT: Gocovri® for the treatment of dyskinesia and OFF episodes
Alex Lowe, Touch Medical Communications Ltd, Stockport, Cheshire, UK
Funding: The publication of this article was supported by Adamas Operations, LLC, a subsidiary of Supernus Pharmaceuticals, Inc who were given the opportunity to review the article for scientific accuracy before submission. Any resulting changes were made at the authors’ discretion.
Introduction
Parkinson’s disease affects approximately 6.1 million people worldwide and 1 million people in the USA.1-3 The condition develops due to loss of nerve cells that produce dopamine in an area of the brain known as the substantia nigra, leading to slow movement, limb rigidity, muscle tremor, and frequent falls.4 Treatment with the dopamine replacement agent, levodopa and other dopamine-based medications typically results in an initial improvement in symptoms.5, 6 However, as Parkinson’s disease progresses, treatment is limited by the emergence of motor complications in nine out of ten patients after 10 years.7 These motor complications can include the development of periods where the response to treatment becomes more variable e.g. the medication effect wears off early, is not working or suddenly loses its effect (termed OFF episodes). Additionally, patients may develop periods of involuntary movements, termed dyskinesia.8-10 Dyskinesias are typically erratic in nature, compared with the rhythmic nature of tremors. Both OFF episodes and dyskinesia adversely impact the daily activities of patients, their quality of life, increase the burden for their care partner(s) and result in additional healthcare costs.11-17
Treatment of OFF episodes and dyskinesia
Traditional approaches to managing OFF episodes, including using higher doses of levodopa or adding other medications, can worsen dyskinesia.18-23 Conversely, lowering doses of these medications to reduce dyskinesia can cause more and/or longer OFF episodes, leading to a dilemma for symptom management.18-23 A medication called amantadine has historically been used as an alternative treatment option. This medication may result in minor improvements in OFF time and dyskinesia. However, the traditional “immediate-release” formulation is limited by its short duration of action resulting in the need for multiple daily doses that can increase the chance of side effects.24-31 Recently, a new amantadine formulation (Gocovri®) has been developed. Gocovri has delayed and extended-release properties that allow it to be taken once daily at bedtime.32 With bedtime administration, the Gocovri formulation allows amantadine levels to slowly rise overnight, providing higher blood drug levels during the waking day and lower drug levels at bedtime.33 Higher daytime amantadine blood levels are thought to be helpful in treating dyskinesia, while lower bedtime levels aim to minimize adverse effects on sleep.34, 35 Gocovri became the first drug with USA regulatory (FDA) approval for the treatment of dyskinesia, and is also approved to treat OFF episodes in patients with Parkinson’s disease taking levodopa.32, 36, 37 This article reviews the key results from studies of Gocovri in Parkinson’s disease. For further information on the clinical development of Gocovri, please click here to view the article.
The clinical evidence for Gocovri in Parkinson’s Disease
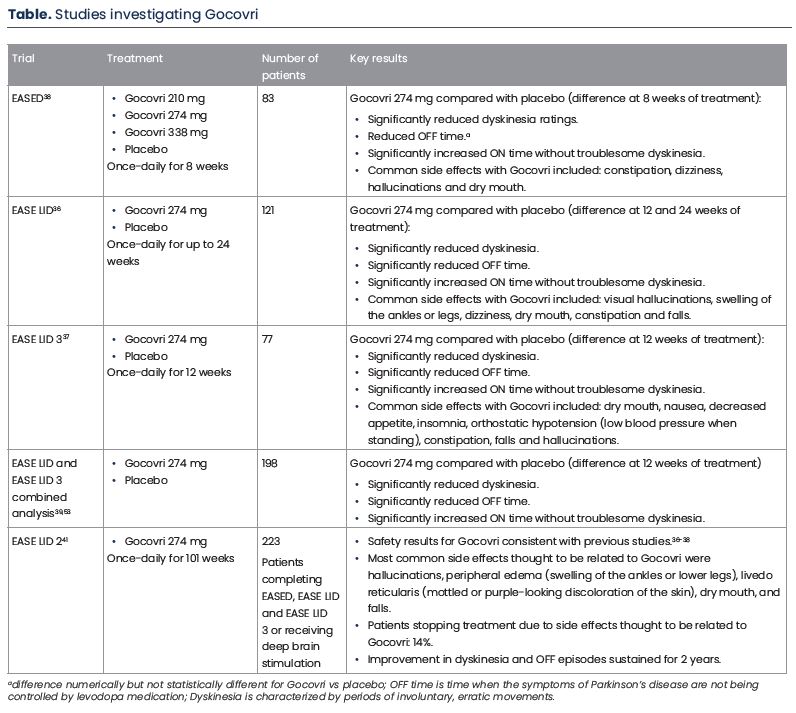
The clinical benefit of Gocovri was primarily determined in three clinical studies including approximately 280 patients with Parkinson’s disease and dyskinesia, receiving levodopa therapy; approximately nine out of ten patients also experienced OFF episodes.36-38 An overview of these studies is included in the Table.
The first study, named EASED compared the effect of three different Gocovri doses (210, 274 and 338 mg) once-daily with a dummy version of the drug, termed placebo, in approximately 80 patients.38 In this study patients receiving Gocovri 274 mg for 8 weeks demonstrated a significant reduction in dyskinesia, the primary endpoint of the study, compared with placebo (Table). Gocovri had acceptable safety, with no effect on sleep duration observed. Common side effects with Gocovri included constipation, dizziness, hallucinations (seeing or hearing things that are not real) and dry mouth.38 As more patients had to withdraw from the study due to side effects with the highest 338 mg Gocovri dose, the 274 mg dose was deemed to provide the best benefit-risk profile, and was used in future studies.
The second and third clinical studies, named EASE LID and EASE LID 3, further investigated Gocovri 274 mg once-daily compared with placebo, in more patients (121 and 77 patients, respectively) and for a longer period of time (up to 24 and 12 weeks, respectively) (Table).36, 37 Gocovri significantly reduced the impact and amount of daily time spent with dyskinesia throughout each of these studies. Additionally, these improvements were coupled with a significant reduction in OFF time. Safety results for Gocovri were generally consistent with those of the previous EASED study. Common side effects with Gocovri included hallucinations, dizziness, dry mouth, constipation, swelling of the legs and ankles, constipation and falls.
Further investigation of Gocovri’s effect
Gocovri’s clinical benefit has been further assessed in several additional analyses and a long-term study.13, 39-41 Analyses using patient data from both the EASE LID and EASE LID 3 studies (almost 200 patients in total),39 showed that reductions in dyskinesia were generally seen within two weeks of Gocovri initiation, with reductions in OFF time compared with placebo seen from 8 weeks of Gocovri initiation.39
A separate analysis of patient diary data available for 162 patients from the EASE LID and EASE LID 3 studies provided a novel examination of the patient experience of Parkinson’s disease symptoms over the course of the day.40 In their diaries for every 30-minute interval throughout the day, patients categorized whether they were predominantly in an OFF state (when Parkinson’s symptoms were present), an ON state (where levodopa and other Parkinson’s medications were working well), or an ON state but with dyskinesia that was sufficient to be considered “troublesome”. The analysis found that patients receiving Gocovri had decreased variability in their day, with approximately 25% fewer transitions between OFF, ON, and dyskinetic states compared with placebo. This resulted in longer daily periods of continuous “good” ON time (without troublesome dyskinesia).40
The final analysis assessed everyday activities that are commonly affected by dyskinesia.13 The aim was to see if Gocovri, by lessening dyskinesia, could improve a patient’s ability to perform or participate in these activities.13 Of the ten activities assessed, six of them were rated as causing mild to severe impairment by the majority of patients. Compared with placebo, Gocovri significantly reduced the impact of dyskinesia on walking and balance, eating, speech, engaging in hobbies, interacting in public or social settings, and when dealing with exciting or emotionally-charged situations. Gocovri also reduced the overall severity of dyskinesia in all body regions assessed.
Long-term effect of Gocovri
The long-term effect of Gocovri was assessed in the 101-week EASE LID 2 study (Table).41 Patients who completed the EASED, EASE LID and EASE LID 3 studies were eligible to receive Gocovri in the EASE LID 2 study. Additionally, patients receiving deep brain stimulation, were allowed to enroll (use of deep brain stimulation was not allowed in the earlier trials). In total, three-quarters of patients completed one year of the study and nearly 60% completed the full two-year study, with only 14% of patients discontinuing treatment due to side effects thought to be Gocovri-related. The safety of Gocovri was consistent with previous studies.36-38 By 8 weeks of the study, on average, patients had less OFF time and dyskinesia compared with before Gocovri initiation, and these improvements were maintained for the 100 weeks of study.
Conclusion
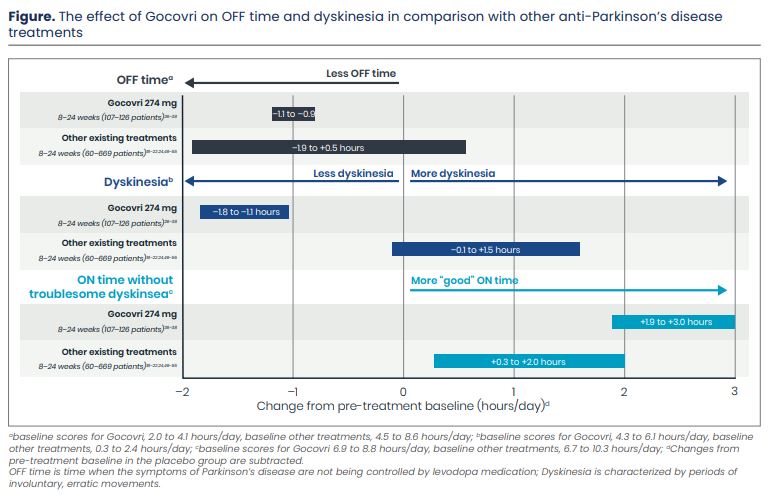
Levodopa is an effective medication to treat Parkinson’s disease; however over time, OFF episodes and dyskinesia can develop. These unwanted motor conditions can interfere with a person’s ability to perform daily activities or participate in social activities. Most existing medications that treat OFF episodes, have the potential to either worsen or cause dyskinesia (Figure).18-22, 24, 37, 38, 42-52 In clinical studies, Gocovri, taken once-daily at bedtime reduced OFF episodes and dyskinesia (Figure). These effects appeared to continue over two years of treatment. Additionally, patients experienced improvement in day-to-day activities typically affected by dyskinesia, had fewer transitions into and out of OFF and dyskinesia episodes, and longer periods of continuous good motor function (“ON” time) without troublesome dyskinesia. Together, these results suggest that Gocovri represents a clinically significant advance in treating dyskinesia and OFF time in Parkinson’s disease.
References
- Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17(11): 939-53.
- Group L. Economic Burden and Future Impact of Parkinson's Disease weblink: https://www.michaeljfox.org/sites/default/files/media/document/2019%20Parkinson%27s%20Economic%20Burden%20Study%20-%20FINAL.pdf (accessed February 2022). 2019.
- Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis 2018; 4: 21.
- DeMaagd G, Philip A. Parkinson's Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. P t 2015; 40(8): 504-32.
- Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol 2020; 27(1): 27-42.
- Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson's disease. Mov Disord 2018; 33(8): 1248-66.
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001; 16(3): 448-58.
- Salat D, Tolosa E. Levodopa in the treatment of Parkinson's disease: current status and new developments. J Parkinsons Dis 2013; 3(3): 255-69.
- Warren Olanow C, Kieburtz K, Rascol O, et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov Disord 2013; 28(8): 1064-71.
- Chou KL, Stacy M, Simuni T, et al. The spectrum of "off" in Parkinson's disease: What have we learned over 40 years? Parkinsonism Relat Disord 2018; 51: 9-16.
- Chapuis S, Ouchchane L, Metz O, et al. Impact of the motor complications of Parkinson's disease on the quality of life. Mov Disord 2005; 20(2): 224-30.
- Hechtner MC, Vogt T, Zollner Y, et al. Quality of life in Parkinson's disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat Disord 2014; 20(9): 969-74.
- Pahwa R, Isaacson S, Jimenez-Shaheed J, et al. Impact of dyskinesia on activities of daily living in Parkinson's disease: Results from pooled phase 3 ADS-5102 clinical trials. Parkinsonism Relat Disord 2019; 60: 118-25.
- Mosley PE, Moodie R, Dissanayaka N. Caregiver Burden in Parkinson Disease: A Critical Review of Recent Literature. J Geriatr Psychiatry Neurol 2017; 30(5): 235-52.
- Onozawa R, Tsugawa J, Tsuboi Y, et al. The impact of early morning off in Parkinson's disease on patient quality of life and caregiver burden. J Neurol Sci 2016; 364: 1-5.
- Suh DC, Pahwa R, Mallya U. Treatment patterns and associated costs with Parkinson's disease levodopa induced dyskinesia. J Neurol Sci 2012; 319(1-2): 24-31.
- Findley LJ, Wood E, Lowin J, et al. The economic burden of advanced Parkinson's disease: an analysis of a UK patient dataset. J Med Econ 2011; 14(1): 130-9.
- Borgohain R, Szasz J, Stanzione P, et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson's disease. Mov Disord 2014; 29(10): 1273-80.
- Ferreira JJ, Lees A, Rocha JF, et al. Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol 2016; 15(2): 154-65.
- Hauser RA, Hubble JP, Truong DD. Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology 2003; 61(3): 297-303.
- Hauser RA, Shulman LM, Trugman JM, et al. Study of istradefylline in patients with Parkinson's disease on levodopa with motor fluctuations. Mov Disord 2008; 23(15): 2177-85.
- Mizuno Y, Nomoto M, Hasegawa K, et al. Rotigotine vs ropinirole in advanced stage Parkinson's disease: a double-blind study. Parkinsonism Relat Disord 2014; 20(12): 1388-93.
- Schaeffer E, Pilotto A, Berg D. Pharmacological strategies for the management of levodopa-induced dyskinesia in patients with Parkinson's disease. CNS Drugs 2014; 28(12): 1155-84.
- Goetz CG, Stebbins GT, Chung KA, et al. Which dyskinesia scale best detects treatment response? Mov Disord 2013; 28(3): 341-6.
- Sawada H, Oeda T, Kuno S, et al. Amantadine for dyskinesias in Parkinson's disease: a randomized controlled trial. PLoS One 2010; 5(12): e15298.
- Snow BJ, Macdonald L, McAuley D, et al. The effect of amantadine on levodopa-induced dyskinesias in Parkinson's disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 2000; 23(2): 82-5.
- Metman LV, Del Dotto P, LePoole K, et al. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol 1999; 56(11): 1383-6.
- Metman VL, Del Dotto P, van den Munckhof P, et al. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease. Neurology 1998; 50(5): 1323-6.
- Thomas A, Iacono D, Luciano AL, et al. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry 2004; 75(1): 141-3.
- Wolf E, Seppi K, Katzenschlager R, et al. Long-term antidyskinetic efficacy of amantadine in Parkinson's disease. Mov Disord 2010; 25(10): 1357-63.
- Ory-Magne F, Corvol JC, Azulay JP, et al. Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology 2014; 82(4): 300-7.
- FDA. GOCOVRI (amantadine) extended release capsules Highlights of prescribing information; Weblink: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208944s003lbl.pdf (accessed February 2022). 2017.
- Hauser RA, Pahwa R, Wargin WA, et al. Pharmacokinetics of ADS-5102 (Amantadine) Extended Release Capsules Administered Once Daily at Bedtime for the Treatment of Dyskinesia. Clin Pharmacokinet 2019; 58(1): 77-88.
- Hayden FG, Gwaltney JM, Jr., Van de Castle RL, et al. Comparative toxicity of amantadine hydrochloride and rimantadine hydrochloride in healthy adults. Antimicrob Agents Chemother 1981; 19(2): 226-33.
- FDA. SYMMETREL® (Amantadine Hydrochloride, USP) weblink: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/016023s041,018101s016lbl.pdf (accessed February 2022). 2009.
- Pahwa R, Tanner CM, Hauser RA, et al. ADS-5102 (Amantadine) Extended-Release Capsules for Levodopa-Induced Dyskinesia in Parkinson Disease (EASE LID Study): A Randomized Clinical Trial. JAMA Neurol 2017; 74(8): 941-9.
- Oertel W, Eggert K, Pahwa R, et al. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson's disease (EASE LID 3). Mov Disord 2017; 32(12): 1701-9.
- Pahwa R, Tanner CM, Hauser RA, et al. Amantadine extended release for levodopa-induced dyskinesia in Parkinson's disease (EASED Study). Mov Disord 2015; 30(6): 788-95.
- Elmer LW, Juncos JL, Singer C, et al. Pooled Analyses of Phase III Studies of ADS-5102 (Amantadine) Extended-Release Capsules for Dyskinesia in Parkinson's Disease. CNS Drugs 2018; 32(4): 387-98.
- Hauser RA, Kremens DE, Elmer LW, et al. Prevalence of Dyskinesia and OFF by 30-Minute Intervals Through the Day and Assessment of Daily Episodes of Dyskinesia and OFF: Novel Analyses of Diary Data from Gocovri Pivotal Trials. J Parkinsons Dis 2019; 9(3): 591-600.
- Tanner CM, Pahwa R, Hauser RA, et al. EASE LID 2: A 2-Year Open-Label Trial of Gocovri (Amantadine) Extended Release for Dyskinesia in Parkinson's Disease. J Parkinsons Dis 2020.
- Clinicaltrials.gov. Efficacy and Safety of Amantadine Hydrogen Chloride (HCl) ER Tablets in Parkinson's Disease Subjects With LID (ALLAY-LID-II) Available at: https://clinicaltrials.gov/ct2/show/results/NCT02153632 (accessed February 2022).
- LeWitt PA, Guttman M, Tetrud JW, et al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces "off" time in Parkinson's disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol 2008; 63(3): 295-302.
- Stacy M, Silver D, Mendis T, et al. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology 2008; 70(23): 2233-40.
- Mizuno Y, Hasegawa K, Kondo T, et al. Clinical efficacy of istradefylline (KW-6002) in Parkinson's disease: a randomized, controlled study. Mov Disord 2010; 25(10): 1437-43.
- Pourcher E, Fernandez HH, Stacy M, et al. Istradefylline for Parkinson's disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism Relat Disord 2012;
18(2): 178-84. - Mizuno Y, Kondo T. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson's disease. Mov Disord 2013; 28(8): 1138-41.
- Zhang Z, Wang J, Zhang X, et al. The efficacy and safety of ropinirole prolonged release tablets as adjunctive therapy in Chinese subjects with advanced Parkinson's disease: a multicenter, double-blind, randomized, placebo-controlled study. Parkinsonism Relat Disord 2013; 19(11): 1022-6.
- Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson's disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2018; 17(9): 749-59.
- Lees AJ, Ferreira J, Rascol O, et al. Opicapone as Adjunct to Levodopa Therapy in Patients With Parkinson Disease and Motor Fluctuations: A Randomized Clinical Trial. JAMA Neurol 2017; 74(2): 197-206.
- Murata M, Hasegawa K, Kanazawa I, et al. Zonisamide improves wearing-off in Parkinson's disease: A randomized, double-blind study. Mov Disord 2015; 30(10): 1343-50.
- Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 2014; 13(2): 141-9.
- Elmer LW, Juncos JL, Singer C, et al. Author Correction to: Pooled Analyses of Phase III Studies of ADS-5102 (Amantadine) Extended-Release Capsules for Dyskinesia in Parkinson's Disease. CNS Drugs 2018; 32(4): 399-400.
